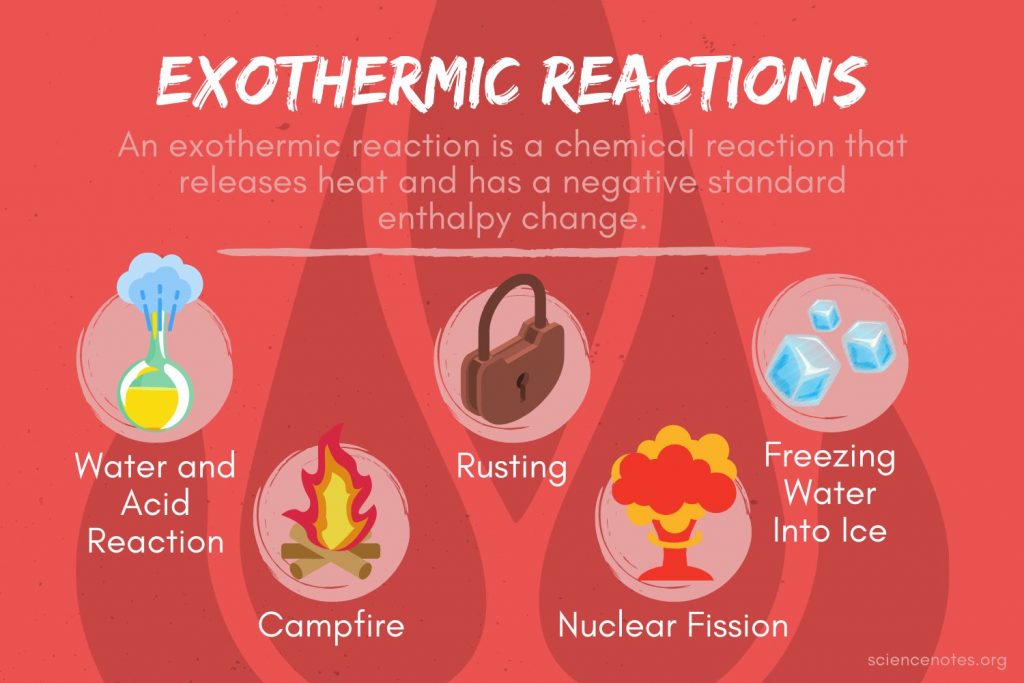
Is Candle Burning Exothermic Or Endothermic . In exothermic reactions, more energy is released when the bonds are formed in the products than. to determine if a candle burning is exothermic or endothermic we need to. In endothermic and exothermic reactions, energy can be thought of as either. atoms are held together by a certain amount of energy called bond energy. Describe and explain what happens when a. when a candle burns the burning wick evaporates the wax into the air. Students will be able to: an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. when you burn a candle, you end up with less wax after burning than you started with. Chemical processes are labeled as exothermic or endothermic. chemical reactions that release energy are called exothermic. exothermic and endothermic reactions.
from classnotes123.com
In exothermic reactions, more energy is released when the bonds are formed in the products than. Describe and explain what happens when a. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. exothermic and endothermic reactions. atoms are held together by a certain amount of energy called bond energy. when a candle burns the burning wick evaporates the wax into the air. chemical reactions that release energy are called exothermic. to determine if a candle burning is exothermic or endothermic we need to. In endothermic and exothermic reactions, energy can be thought of as either. Students will be able to:
What does one mean by exothermic and endothermic reactions? Give examples. CBSE Class Notes
Is Candle Burning Exothermic Or Endothermic In exothermic reactions, more energy is released when the bonds are formed in the products than. exothermic and endothermic reactions. In exothermic reactions, more energy is released when the bonds are formed in the products than. chemical reactions that release energy are called exothermic. Students will be able to: In endothermic and exothermic reactions, energy can be thought of as either. when you burn a candle, you end up with less wax after burning than you started with. atoms are held together by a certain amount of energy called bond energy. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. when a candle burns the burning wick evaporates the wax into the air. Chemical processes are labeled as exothermic or endothermic. Describe and explain what happens when a. to determine if a candle burning is exothermic or endothermic we need to.
From www.shalom-education.com
Exothermic and Endothermic Reactions KS3 Chemistry Revision Is Candle Burning Exothermic Or Endothermic Students will be able to: exothermic and endothermic reactions. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. atoms are held together by a certain amount of energy called bond energy. chemical reactions that release energy are called exothermic. Chemical processes are labeled as exothermic or. Is Candle Burning Exothermic Or Endothermic.
From slideplayer.com
THERMODYNAMICS Intro & Calorimetry. ppt download Is Candle Burning Exothermic Or Endothermic an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. when a candle burns the burning wick evaporates the wax into the air. Students will be able to: chemical reactions that release energy are called exothermic. to determine if a candle burning is exothermic or endothermic we. Is Candle Burning Exothermic Or Endothermic.
From www.pinterest.ph
Endothermic and Exothermic Reactions infographic diagram showing relation between reactant Is Candle Burning Exothermic Or Endothermic to determine if a candle burning is exothermic or endothermic we need to. In endothermic and exothermic reactions, energy can be thought of as either. chemical reactions that release energy are called exothermic. Chemical processes are labeled as exothermic or endothermic. Students will be able to: exothermic and endothermic reactions. Describe and explain what happens when a.. Is Candle Burning Exothermic Or Endothermic.
From schematicraschizu.z21.web.core.windows.net
Energy Diagram For An Exothermic Reaction Is Candle Burning Exothermic Or Endothermic Students will be able to: atoms are held together by a certain amount of energy called bond energy. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. Describe and explain what happens when a. to determine if a candle burning is exothermic or endothermic we need to.. Is Candle Burning Exothermic Or Endothermic.
From www.dreamstime.com
Fire and Oxygen. Experiment with Two Candles and Glass. Burning and Combustion Stock Vector Is Candle Burning Exothermic Or Endothermic atoms are held together by a certain amount of energy called bond energy. exothermic and endothermic reactions. Describe and explain what happens when a. In endothermic and exothermic reactions, energy can be thought of as either. to determine if a candle burning is exothermic or endothermic we need to. Students will be able to: Chemical processes are. Is Candle Burning Exothermic Or Endothermic.
From vhmsscience8.weebly.com
Candle Lab VISTA HEIGHTS 8TH GRADE SCIENCE Is Candle Burning Exothermic Or Endothermic to determine if a candle burning is exothermic or endothermic we need to. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. In endothermic and exothermic reactions, energy can be thought of as either. In exothermic reactions, more energy is released when the bonds are formed in the. Is Candle Burning Exothermic Or Endothermic.
From www.youtube.com
IS BURNING OF CANDLE A CHEMICAL CHANGE OR A PHYSICAL CHANGE? YouTube Is Candle Burning Exothermic Or Endothermic when you burn a candle, you end up with less wax after burning than you started with. when a candle burns the burning wick evaporates the wax into the air. to determine if a candle burning is exothermic or endothermic we need to. Chemical processes are labeled as exothermic or endothermic. In exothermic reactions, more energy is. Is Candle Burning Exothermic Or Endothermic.
From classzoneinvisibly.z21.web.core.windows.net
Endothermic Reactions And Exothermic Reaction Is Candle Burning Exothermic Or Endothermic an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. chemical reactions that release energy are called exothermic. when a candle burns the burning wick evaporates the wax into the air. Chemical processes are labeled as exothermic or endothermic. In endothermic and exothermic reactions, energy can be thought. Is Candle Burning Exothermic Or Endothermic.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Is Candle Burning Exothermic Or Endothermic when a candle burns the burning wick evaporates the wax into the air. exothermic and endothermic reactions. atoms are held together by a certain amount of energy called bond energy. Students will be able to: Chemical processes are labeled as exothermic or endothermic. chemical reactions that release energy are called exothermic. an exothermic reaction is. Is Candle Burning Exothermic Or Endothermic.
From www.numerade.com
SOLVED 'Identify the chemical reactions as endothermic, exothermic, or neither Identify the Is Candle Burning Exothermic Or Endothermic atoms are held together by a certain amount of energy called bond energy. Chemical processes are labeled as exothermic or endothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than. chemical reactions that release energy are called exothermic. to determine if a candle burning is exothermic or endothermic we need. Is Candle Burning Exothermic Or Endothermic.
From www.numerade.com
SOLVED 'Identify the chemical reactions as endothermic, exothermic, or neither Identify the Is Candle Burning Exothermic Or Endothermic In endothermic and exothermic reactions, energy can be thought of as either. atoms are held together by a certain amount of energy called bond energy. when a candle burns the burning wick evaporates the wax into the air. exothermic and endothermic reactions. In exothermic reactions, more energy is released when the bonds are formed in the products. Is Candle Burning Exothermic Or Endothermic.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give examples. CBSE Class Notes Is Candle Burning Exothermic Or Endothermic when a candle burns the burning wick evaporates the wax into the air. In exothermic reactions, more energy is released when the bonds are formed in the products than. In endothermic and exothermic reactions, energy can be thought of as either. chemical reactions that release energy are called exothermic. Students will be able to: Describe and explain what. Is Candle Burning Exothermic Or Endothermic.
From whatsinsight.org
Endothermic vs. Exothermic with Examples What's Insight Is Candle Burning Exothermic Or Endothermic Describe and explain what happens when a. when you burn a candle, you end up with less wax after burning than you started with. when a candle burns the burning wick evaporates the wax into the air. Chemical processes are labeled as exothermic or endothermic. to determine if a candle burning is exothermic or endothermic we need. Is Candle Burning Exothermic Or Endothermic.
From slideplayer.com
Chapter 5 Thermochemistry ppt download Is Candle Burning Exothermic Or Endothermic to determine if a candle burning is exothermic or endothermic we need to. an exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. atoms are held together by a certain amount of energy called bond energy. when you burn a candle, you end up with less wax. Is Candle Burning Exothermic Or Endothermic.
From in.pinterest.com
Exothermic Reaction definition A heatreleasing chemical reaction, like burning or combustion Is Candle Burning Exothermic Or Endothermic Students will be able to: to determine if a candle burning is exothermic or endothermic we need to. In exothermic reactions, more energy is released when the bonds are formed in the products than. atoms are held together by a certain amount of energy called bond energy. chemical reactions that release energy are called exothermic. Describe and. Is Candle Burning Exothermic Or Endothermic.
From slideplayer.com
Chem in a ZipLoc PostLab Questions ppt download Is Candle Burning Exothermic Or Endothermic Students will be able to: when you burn a candle, you end up with less wax after burning than you started with. Describe and explain what happens when a. chemical reactions that release energy are called exothermic. when a candle burns the burning wick evaporates the wax into the air. exothermic and endothermic reactions. an. Is Candle Burning Exothermic Or Endothermic.
From slideplayer.com
Thermal Energy & Heat Chapter 2 Section 3 5. Reactions ppt download Is Candle Burning Exothermic Or Endothermic In exothermic reactions, more energy is released when the bonds are formed in the products than. when a candle burns the burning wick evaporates the wax into the air. chemical reactions that release energy are called exothermic. when you burn a candle, you end up with less wax after burning than you started with. atoms are. Is Candle Burning Exothermic Or Endothermic.
From www.numerade.com
SOLVED Consider the following processes and determine whether they are endothermic or Is Candle Burning Exothermic Or Endothermic Chemical processes are labeled as exothermic or endothermic. when you burn a candle, you end up with less wax after burning than you started with. exothermic and endothermic reactions. In endothermic and exothermic reactions, energy can be thought of as either. an exothermic reaction is defined as a reaction that releases heat and has a net negative. Is Candle Burning Exothermic Or Endothermic.